

According to reports, Phinergy expects this technology to be used in a commercial vehicle by 2017. The vehicle functioned like a hybrid by using the aluminum-air battery to extend its driving range. In March 2013, Phinergy, a developer of aluminum-air battery technology, test-drove a Citroën C1 (city car or mini) that was powered by a small lithium-ion battery and fitted with a 50-plate (55-lb) aluminum-air battery that charged the lithium-ion battery. The goal of this study was to develop a high-capacity rechargeable aluminumair battery with resistance toward the degradation induced by long-term chargedischarge electrochemical reactions. See also: Aluminum (metallurgy) Wide-scale infrastructure for electric cars However, the by-product of the battery’s reaction, aluminum hydroxide, is recyclable. Once the aluminum is consumed, the battery must be replaced, which means that some sort of battery-swapping station or service would need to be readily available to drivers. By comparison, the Nissan Leaf’s 600-lb (270-kg) lithium-ion battery pack provides a driving range of about 100 mi (160 km).Ī major disadvantage of the aluminum-air battery is that it is not rechargeable. It is estimated that 55 lb (25 kg) of aluminum will provide a driving range of about 1000 mi (1600 km) and the entire battery will weigh about 200 lb (90 kg). See also: Aluminum Electricity Electrolyte Oxidation-reduction Oxideīecause aluminum is a lightweight metal and the cathode material, oxygen, does not have to be stored in the battery, an aluminum-air battery is considerably lighter than a comparable lithium-ion battery.
Cathode reaction of aluminum air battery plus#
When the battery is in use, the oxidation reaction of aluminum plus oxygen plus water produces aluminum hydroxide plus electrical energy (electrons). A selectively permeable membrane allows oxygen from the air to enter the cell but excludes gases such as carbon dioxide that would interfere with the battery’s function. The aluminum-air battery consists of an aluminum anode in an electrolyte solution of potassium hydroxide and uses oxygen from air as the cathode. In 2013, an aluminum-air battery was demonstrated that could extend the driving range of an electric vehicle up to 1000 mi (1600 km).
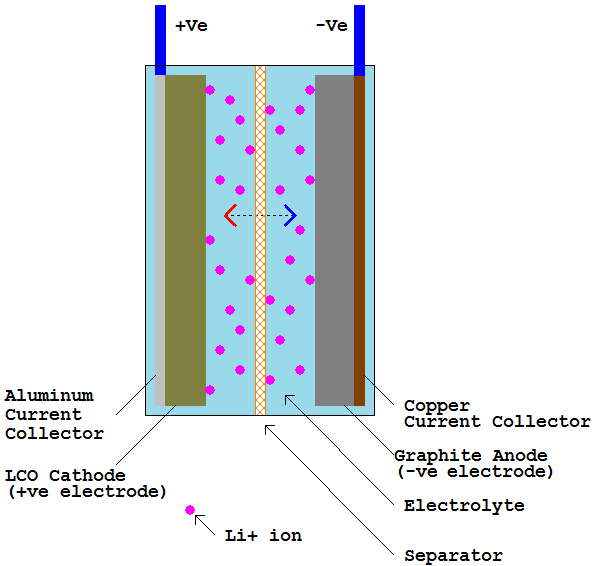
In addition to being slow to charge, the lithium-ion battery packs that power EVs are heavy and expensive, and typically will need to be replaced at least once during the vehicles’ lifetime.
Cathode reaction of aluminum air battery drivers#
After that, drivers have to plug into a 240-volt charger for six to eight hours for a full charge, find a quick-charge station and plug into a 480-volt charger for 20 to 30 minutes for 80% of a full charge, or call a tow truck. Advances in aluminum-air battery technology might allow electrically powered vehicles to become more realistic and competitive automotive options for many drivers.Īt present, most electric vehicles (EVs) are powered by lithium-ion battery packs and have a driving range of about 100 miles (160 km).


 0 kommentar(er)
0 kommentar(er)
